Aptamer-mediated selective delivery of a cytotoxic cationic NHC-Au(i) complex to cancer cells†
Abstract
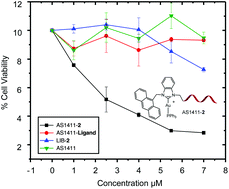
A novel cationic NHC-Au(I) complex was synthesized and studied for its antitumor activity. For all the cell lines tested, cationic NHC-Au(I) complex 2 shows much higher cytotoxicity than its neutral analogue 1. To achieve selective cancer cell targeting, complex 2 was covalently conjugated to aptamer AS1411, a DNA aptamer with strong binding affinity for nucleolin. The successful conjugation was confirmed by HPLC, gel electrophoresis, fluorescence spectroscopy and UV-Vis absorption. Conjugate AS1411-2 was then examined for its specific targeting and binding ability towards cancer cells over human normal cells using flow cytometry analysis and confocal microscopy. The cytotoxicity of AS1411-2 was then estimated by MTS assay. It was found that AS1411-2 exhibits higher activity than complex 2 towards targeted cells. Importantly, AS1411-2 exhibits much lower cytotoxicity towards healthy normal cell lines. Concurrently, the control groups without the AS1411 aptamer or without the NHC-Au(I) complex do have significant impact on cancer cell viability.


 Please wait while we load your content...
Please wait while we load your content...