The effect of electrostatic field on the catalytic properties of platinum clusters confined in zeolite for hydrogenation†
Abstract
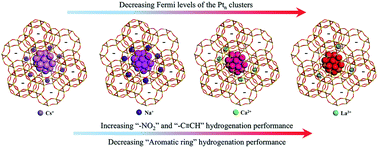
The electrostatic field of a dielectric cavity is powerful for modulating many aspects of confined metal clusters. Here we report that the catalytic properties of Ptn clusters confined in X-zeolite cages are strongly tuned by altering the zeolitic field, which is achieved by using different cations (Na+, Cs+, Ca2+ or La3+) to balance the negative framework of the zeolite. Compared with Ptn@LaX, for example, Ptn@CsX shows a surprising ∼5500% enhancement in TOF value for phenol hydrogenation to cyclohexanol. Electrostatic field modulation leads to gradual changes of electronic properties such as the Fermi levels/d-band centers of the Ptn clusters, as revealed by DFT calculations and experimental characterizations. The decreases in Fermi levels of the Ptn clusters benefit the hydrogenation of –C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CH groups (phenylacetylene and acetylene) and –NO2 groups (nitrobenzene, o-nitroanisole and o-chloronitrobenzene) but are detrimental for aromatic ring hydrogenation (phenol). These findings are important for the design of high performance catalysts.
CH groups (phenylacetylene and acetylene) and –NO2 groups (nitrobenzene, o-nitroanisole and o-chloronitrobenzene) but are detrimental for aromatic ring hydrogenation (phenol). These findings are important for the design of high performance catalysts.


 Please wait while we load your content...
Please wait while we load your content...