C3N4–Mn/CNT composite as a heterogeneous catalyst in the electro-peroxone process for promoting the reaction between O3 and H2O2 in acid solution†
Abstract
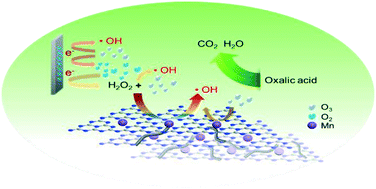
The electro-peroxone (E-P) process normally exhibits low efficiency in organic degradation in acid solution due to the limited rate constant of the reaction between O3 and H2O2 (peroxone reaction). In this paper, a C3N4–Mn/CNT composite material was synthetized and characterized with XRD, FTIR, SEM and XPS. With the function of the C3N4–Mn/CNT catalyst, the substrate oxalic acid (OA) was completely degraded in the E-P process within 30 min at pH 3, while only 15% degradation could be achieved after 60 min without the catalyst. The comparison of different reaction systems using the C3N4–Mn/CNT catalyst confirmed that the catalyst promoted the peroxone reaction, rather than the catalytic ozonation or electro-Fenton like reaction. Correlating the XRD and XPS analysis with the degradation data using the C3N4–Mn/CNT catalyst, we deduced that Mn coordinated with nitrogen was the active site for the peroxone reaction. Furthermore, the catalyst showed good chemical and catalytic stability in five cycles of the E-P process for OA degradation.


 Please wait while we load your content...
Please wait while we load your content...