Encapsulation of laccase within zwitterionic poly-carboxybetaine hydrogels for improved activity and stability
Abstract
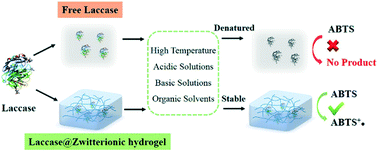
Laccase is an important oxidase for many applications such as bioremediation, chemical synthesis, and biosensors, due to its wide range of substrates and prominent capacity to degrade phenolic compounds. However, its unsatisfactory stability, activity, and reusability still remain a major challenge for practical applications. In this work, we found that when laccase was encapsulated in zwitterionic hydrogels, especially poly-carboxybetaine (PCB), its performance could be significantly enhanced. Compared with the free enzyme, PCB–laccase displayed a remarkably increased activity (∼20-fold higher Kcat/Km value). Meanwhile, its stability could also be effectively maintained even in an extensive range of temperatures, pH values and organic solvents. In addition, PCB–laccase demonstrated excellent storage stability, and retained 80% of its initial activity after an 8-week storage period. In a successive 6-cycle decoloration of acid orange 7, PCB–laccase could still retain 52% of its initial activity, demonstrating superior durability to the free laccase. Therefore, we have provided an efficient and stable support matrix for enzyme immobilization, which exhibits potential for practical applications even in harsh environments.


 Please wait while we load your content...
Please wait while we load your content...