Structure-guided engineering of meso-diaminopimelate dehydrogenase for enantioselective reductive amination of sterically bulky 2-keto acids†
Abstract
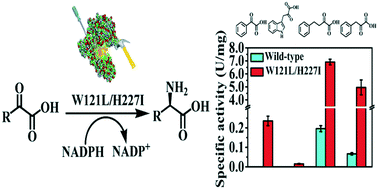
meso-Diaminopimelate dehydrogenase (DAPDH) and mutant enzymes are an excellent choice of biocatalysts for the conversion of 2-keto acids to the corresponding D-amino acids. However, their application in the enantioselective reductive amination of bulky 2-keto acids, such as phenylglyoxylic acid, 2-oxo-4-phenylbutyric acid, and indole-3-pyruvic acid, is still challenging. In this study, the structure-guided site-saturation mutagenesis of a Symbiobacterium thermophilum DAPDH (StDAPDH) gave rise to a double-site mutant W121L/H227I, which showed dramatically improved enzyme activities towards various 2-keto acids including these sterically bulky substrates. Several D-amino acids were prepared in optically pure form. The molecular docking of substrates into the active sites of wild-type and mutant W121L/H227I enzymes revealed that the substrate binding cavity of the mutant enzyme was reshaped to accommodate these bulky substrates, thus leading to higher enzyme activity. These results lay a foundation for further shaping the substrate binding pocket and manipulating the interactions between the substrate and binding sites to access highly active D-amino acid dehydrogenases for the preparation of synthetically challenging D-amino acids.


 Please wait while we load your content...
Please wait while we load your content...