Oxidative functionalization of C–H compounds induced by the extremely efficient osmium catalysts (a review)
Abstract
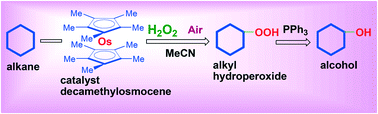
Osmium derivatives are not as popular among catalytic chemists as the compounds of iron (an analog of osmium), copper, or manganese. Although osmium is expensive, poisonous and volatile, it finds applications not only in cis-hydroxylation of olefins but its derivatives have recently been employed in the oxygenation of C–H compounds (saturated and aromatic hydrocarbons and alcohols) by hydrogen peroxide as well as organic peroxides. The turnover in alkane oxidations catalyzed by soluble osmium derivatives has been found to be much higher than analogous reactions that used complexes of other transition metals (e.g., iron, manganese or copper). Using certain additives, the authors of this review have developed osmium-containing systems that are extremely efficient in transforming alkanes into corresponding alkyl hydroperoxides, which can further be reduced to alcohols easily. The new catalytic systems described here are capable of producing ground-breaking results in the field of metal-complex catalysts.


 Please wait while we load your content...
Please wait while we load your content...