Absorbed hydrogen enhances the catalytic hydrogenation activity of Rh-based nanocatalysts†
Abstract
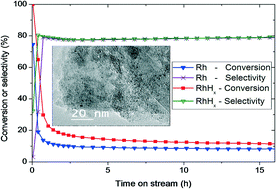
Carbon-supported Rh-based nanoparticles with an average size of 1.0 nm have been tested for the hydrogenation of butadiene in two different forms: either metal Rh or hydride RhHx nanoparticles. Laboratory tests demonstrated that the Rh hydride nanocatalyst is more active than its metal counterpart, irrespective of the gas feed composition and temperature. Moreover, this difference is significantly more important at the initial stage of the reaction compared to that under quasi-stationary conditions. However, the reaction mechanisms appear similar for the metal and hydride nanocatalysts, as suggested by the similar selectivities to butenes, apparent reaction energies, and reaction orders. The local structures of both Rh and RhHx nanocatalysts were studied by operando XAS under stationary conditions. EXAFS analyses confirm that the RhHx and Rh catalysts preserve their structure during the reaction as either the hydride or metal phase, respectively. We suggest that the Mars–van Krevelen mechanism might occur at the initial stage but becomes progressively less predominant. Under quasi-stationary conditions, only electronic effects are invoked to explain the activity difference between the hydride and the metal phases. We hypothesize that the stabilization of Rh–H bonds at the surface in the presence of subsurface hydrogen, consistent with previous theoretical findings, explains the higher activity of the hydride catalyst.


 Please wait while we load your content...
Please wait while we load your content...