CO oxidation on SnO2 surfaces enhanced by metal doping†
Abstract
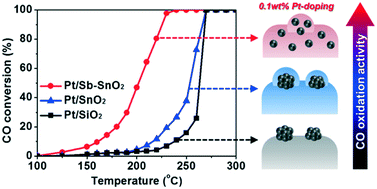
Doping metal atoms into a host metal oxide lattice can enhance its catalytic activity by modulating the properties of surface oxygen. Here, Pt-doped antimony–tin oxide (Pt/Sb–SnO2) was compared with Pt-deposited tin oxide (Pt/SnO2) and Pt-deposited silica (Pt/SiO2) for the oxidation of CO and propylene. 0.1 wt% Pt was deposited in all three cases. High angle annular dark field scanning transmission electron microscopy images, diffuse reflectance infrared Fourier transform spectra, pulsed H2 chemisorption results, and X-ray photoelectron spectra indicated that Pt/Sb–SnO2 has atomically doped Pt inside the SnO2 lattice while Pt/SnO2 has Pt nanoparticles covered with SnO2 layers and Pt/SiO2 has Pt nanoparticles exposed at the SiO2 surface. Pt/Sb–SnO2 showed the best activity for CO oxidation but the poorest activity for propylene oxidation. Propylene oxidation occurred the least on Pt/Sb–SnO2 due to the lack of surface Pt sites. CO temperature-programmed reduction and O2 temperature-programmed desorption results revealed that surface oxygen is the most active on Pt/Sb–SnO2. The formation of carbonates during CO oxidation was monitored, and Pt/Sb–SnO2 showed the least amount of surface carbonates with enhanced activity and durability. Doping a minimal amount of precious metal can be an efficient strategy to control the properties of metal oxide catalysts.


 Please wait while we load your content...
Please wait while we load your content...