Reactions of secondary propargylamines with heteroallenes for the synthesis of diverse heterocycles
Abstract
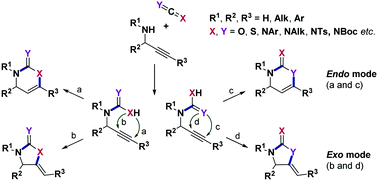
This focused review aims to summarize recent developments in the processes involving additions of secondary propargylamines to various heteroallenes and subsequent transition metal-catalyzed or electrophile-mediated cyclizations. The utility of this convenient and tunable strategy spans from the carbon dioxide fixation and target-oriented synthesis of complex natural and biologically active products to the generation of extended synthetic libraries of diverse oxygen-, nitrogen- and sulfur-containing heterocycles. For comparative purposes, the analogous transformations of propargylic alcohols are also highlighted in this account.


 Please wait while we load your content...
Please wait while we load your content...