Insights into the kinetics of thermally induced crystallization of amorphous calcium phosphate
Abstract
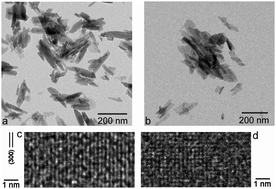
Transformations between amorphous and crystalline apatite mechanistically govern some of the most essential processes in bone metabolism, including biomineralization and bone remodeling. Fundamental understanding of this phase transition can help us gain control over the formation and dissolution of boney tissues in vivo and utilize that knowledge for various therapeutic ends. Crystallization of hydroxyapatite (HAp) and two tricalcium phosphate (TCP) polymorphs from the metastable precursor, amorphous calcium phosphate (ACP) was here studied kinetically and mechanistically using thermal analyses, X-ray diffraction and Fourier-transform infrared spectroscopy. Crystallization was detected in the differential thermal analysis as the exothermic peak at 639.5 °C at the slowest heating regimen of 5 °C min−1, while a combination of different kinetics models, including Augis–Bennett, Borchardt–Daniels, Johnson–Mehl–Avrami, Kissinger, Ozawa and Piloyan, yielded activation energies in the 435–450 kJ mol−1 range. Dehydrated ACP required a significant energy input to transform to HAp, thus indirectly proving the key role that structural water plays in this process in a biological setting. The phase transformation at high temperatures involved preformed nuclei and was solely due to their 3D growth, contrasting the edge-controlled nucleation derived earlier as the mechanism of growth in the solution. Crystallization was in both cases accompanied by the formation of needle-shape crystals of HAp through aggregation of ultrafine spherical units of ACP. Relationship between crystallinity and the heating rate was detected only for the initially amorphous structure, indicating a more intense and coherent lattice ordering process in annealed ACP than in HAp. Despite that, crystallization disobeyed the rule of inverse proportionality between the thermal energy required for the relaxation of defects and the level of strain, as the recovery rate of the initially poorly crystalline HAp was higher than that of ACP.


 Please wait while we load your content...
Please wait while we load your content...