Exposing catalytic versatility of GTPases: taking reaction detours in mutants of hGBP1 enzyme without additional energetic cost†
Abstract
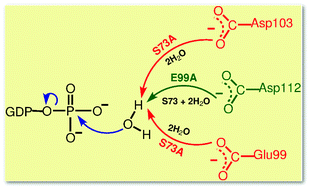
The catalytic power of enzymes is usually ascribed to a few catalytically competent residues as revealed by site-directed mutagenesis studies in conjunction with biochemical, thermodynamic and structural analyses. Surprisingly, the mutations of such pivotal residues in a GTPase that can hydrolyse GTP even to GMP, namely hGBP1, have been reported to result only in marginal changes of the catalytic rate compared to the wild type. Our large-scale ab initio quantum-mechanical/molecular-mechanical (QM/MM) metadynamics simulations disclose that the replacement of catalytically competent residues by the inert amino acid alanine, S73A and E99A, opens a plethora of molecularly different reaction pathways featuring very similar energy barriers and thus rates. These hitherto unknown reaction channels are established by mechanistically involving far-distant residues using “floating” water molecules, which connect them via hydrogen-bonding bridges to the nucleophilic water molecule, thus allowing for efficient long-distance proton transfer via the Grotthuss mechanism. Given the generic nature of the disclosed detour mechanisms that provide the molecular underpinning of catalytic versatility and thus mutational robustness of hGBP1, it is expected that the same concept is operational for GTPases in a broad sense.


 Please wait while we load your content...
Please wait while we load your content...