Influence of chelate ring type on chelate–chelate and chelate–aryl stacking: the case of nickel bis(dithiolene)†
Abstract
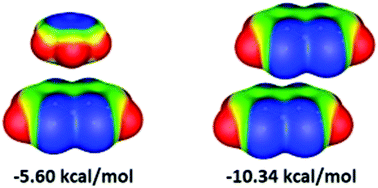
Chelate–aryl and chelate–chelate stacking interactions of nickel bis(dithiolene) were studied at the CCSD(T)/CBS and DFT levels. The strongest chelate–aryl stacking interaction between nickel bis(dithiolene) and benzene has a CCSD(T)/CBS stacking energy of −5.60 kcal mol−1. The strongest chelate–chelate stacking interactions between two nickel bis(dithiolenes) has a CCSD(T)/CBS stacking energy of −10.34 kcal mol−1. The most stable chelate–aryl stacking has the benzene center above the nickel atom, while the most stable chelate–chelate dithiolene stacking has the chelate center above the nickel atom. Comparison of chelate–aryl stacking interactions of dithiolene and acac-type nickel chelate shows similar strength. However, chelate–chelate stacking is stronger for dithiolene nickel chelate than for acac-type nickel chelate, which has a CCSD(T)/CBS interaction energy of −9.50 kcal mol−1.


 Please wait while we load your content...
Please wait while we load your content...