N–H bond activation in ammonia by TM-SSZ-13 (Fe, Co, Ni and Cu) zeolites: a first-principles calculation†
Abstract
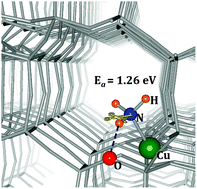
N–H activation is crucial for NH3-assisted selective catalytic reduction (SCR) and selective catalytic oxidation (SCO) of NH3 in exhaust emission treatment of the diesel engine. Copper-exchanged CHA-type zeolites show high performance for N–H activation, however, the atomic-level understanding of the activation mechanism is inaccessible. In this work, we use density functional theory (DFT) calculations to investigate the origin of high activity through electronic structures, magnetic properties and the N–H bond of NH3 activation on the naked Cu2+ as the active site in Cu-SSZ-13. Fe, Co and Ni-SSZ-13 were also systematically studied for comparison. The incorporation of transition metal ions leads to high-spin d-states around the Fermi level. The magnetic moment of 3.858, 2.889, 1.920 and 0.946 μB is observed for Fe, Co, Ni, and Cu-SSZ-13, respectively. A distorted square planar crystal field is found. For Cu-SSZ-13, the electronic configuration of Cu2+ ensures the lowest unoccupancy of b1g (dx2−y2), enabling to receive electrons from base NH3 and thus activate the N–H bond with a barrier of 1.26 eV significantly lower than that in Fe, Co and Ni-SSZ-13. These findings may provide fundamental insights into the high activity and selectivity of Cu-SSZ-13 catalysts for NOx removal and NH3 oxidation at high temperature.


 Please wait while we load your content...
Please wait while we load your content...