Adsorption and oxidation of propane and cyclopropane on IrO2(110)†
Abstract
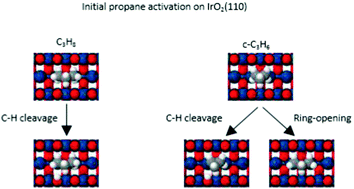
We investigated the adsorption and oxidation of n-propane and cyclopropane (C3H8 and c-C3H6) on the IrO2(110) surface using temperature programmed reaction spectroscopy (TPRS) and density functional theory (DFT) calculations. We find that the activation of both C3H8 and c-C3H6 is facile on IrO2(110) at low temperature, and that the dissociated alkanes oxidize during TPRS to produce CO, CO2 and H2O above ∼400 K. Propane conversion to propylene is negligible during TPRS for the conditions studied. Our results show that the maximum yield of alkane that oxidizes during TPRS is higher for c-C3H6 compared with C3H8 (∼0.30 vs. 0.18 monolayer) and that pre-hydrogenation of the surface suppresses c-C3H6 oxidation to a lesser extent than C3H8. Consistent with the experimental results, DFT predicts that C3H8 and c-C3H6 form σ-complexes on IrO2(110) and that C–H bond activation of the complexes as well as subsequent dehydrogenation are highly facile via H-transfer to Obr atoms (bridging O-atoms). Our calculations predict that propane conversion to gaseous propylene is kinetically disfavored on IrO2(110) because HObr recombination makes Obr atoms available to promote further dehydrogenation at lower temperatures than those needed for the adsorbed C3H6 intermediate to desorb as propylene. We also present evidence that that the ability for c-C3H6 to activate via ring-opening is responsible for cyclopropane attaining higher reaction yields during TPRS and exhibiting a weaker sensitivity to surface pre-hydrogenation compared with n-propane.


 Please wait while we load your content...
Please wait while we load your content...
