Kinetics of the reaction of the simplest Criegee intermediate with ammonia: a combination of experiment and theory†
Abstract
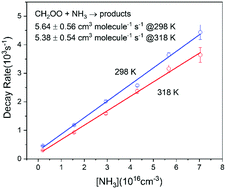
The kinetics of the reaction of the simplest Criegee intermediate (CH2OO) with ammonia has been measured under pseudo-first-order conditions with two different experimental methods. We investigated the rate coefficients at 283, 298, 308, and 318 K at a pressure of 50 Torr using an OH laser-induced fluorescence (LIF) method. Weak temperature dependence of the rate coefficient was observed, which is consistent with the theoretical activation energy of −0.53 kcal mol−1 predicted by quantum chemistry calculation at the QCISD(T)/CBS//B3LYP/6-311+G(2d,2p) level. At 298 K, the rate coefficient at 50 Torr from the OH LIF experiment was (5.64 ± 0.56) × 10−14 cm3 molecule−1 s−1 while at 100 Torr we obtained a slightly larger value of (8.1 ± 1.0) × 10−14 cm3 molecule−1 s−1 using the UV transient absorption method. These experimental values are within the theoretical error bars of the present as well as previous theoretical results. Our experimental results confirmed the previous conclusion that ammonia is negligible in the consumption of CH2OO in the atmosphere. We also note that CH2OO may compete with OH in the oxidation of ammonia under certain circumstances, such as at night-time, high altitude and winter time.
- This article is part of the themed collection: 2018 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...