The effect of electrostatic interactions on the formation of pharmaceutical eutectics
Abstract
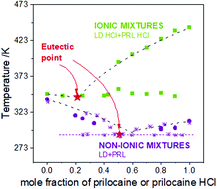
Over the past decade, the formation of pharmaceutical eutectics has become a very attractive strategy to increase the bioavailability of active pharmaceutical ingredients (APIs). A great advantage of a eutectic phase, which can be obtained by simple physical mixing of solid materials, is the possibility to obtain a material with desired physicochemical properties only by varying the molar ratio of the parent components. In this work, we have investigated the ability of two protic ionic liquids (PILs), which are hydrochloride salts of lidocaine and prilocaine, as well as their non-ionic counterparts, to form eutectic mixtures. To gain an insight into the calorimetric properties of the formed dipolar and ionic mixtures, differential scanning calorimetry was employed. The mechanism of formation of deep eutectic mixtures on the molecular level was investigated by ab initio quantum mechanics calculations. The effect of electrostatic interactions on the eutectic transition, glass forming ability and the physical stability of pharmaceutical eutectics was also revealed.


 Please wait while we load your content...
Please wait while we load your content...