Nitrogen matters: the difference between PANH and PAH formation†
Abstract
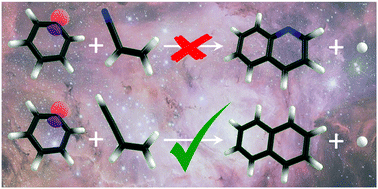
A solid understanding of and a reliable model for the formation pathways of polycyclic aromatic (nitrogen containing) hydrocarbons (PA(N)Hs) is needed to account for their abundance in the interstellar medium and in Titan's atmosphere as well as to mitigate the emission of these carcinogens in our terrestrial environment. We have investigated the phenyl + acrylonitrile reaction mechanism between 600 and 1200 K in a hot microreactor. Radical intermediates (C9H8N˙), formed by addition, and closed-shell C9H7N products, formed by subsequent hydrogen elimination, are isomer-selectively identified using photoion mass-selected threshold photoelectron spectroscopy in conjunction with Franck–Condon simulations. Although quinoline is the most stable product, the calculated potential energy surface and a kinetic model confirm that the reaction is kinetically controlled and yields four open-chain isomers instead. The absence of quinoline is in stark contrast with the isoelectronic phenyl + vinylacetylene reaction that produces naphthalene. Ab initio calculations suggest that this change is brought about by the stability of the nitrile group, which inhibits ring formation. Therefore, it is unlikely that nitrile precursors form nitrogen-containing rings, which calls for alternative pathways by which nitrogen atoms can be incorporated in aromatic systems to explain their presence in the ISM and Titan's atmosphere.


 Please wait while we load your content...
Please wait while we load your content...