Thermally induced carbonation of Ca(OH)2 in a CO2 atmosphere: kinetic simulation of overlapping mass-loss and mass-gain processes in a solid–gas system†
Abstract
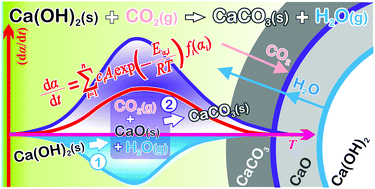
Thermally induced carbonation of Ca(OH)2 in a CO2 atmosphere is a reaction exhibiting particular features, including stoichiometric completeness to form CaCO3 and a kinetic advantage over the carbonation of CaO particles. This study aims to gain further insight into the reaction mechanisms of CO2 capture by Ca(OH)2 and CaO. It focuses on the kinetic modeling of the carbonation of Ca(OH)2 as a consecutive reaction in a solid–gas system. The kinetic behaviors of the thermal decomposition of Ca(OH)2 in an inert gas atmosphere and of the overall process of thermally induced carbonation of Ca(OH)2 in a CO2 atmosphere were investigated using thermal analyses and other complementary techniques. Based on kinetic results, the overall reaction of the thermally induced carbonation of Ca(OH)2 in a CO2 atmosphere was separated by a kinetic deconvolution analysis into two consecutive reaction steps: the thermal decomposition of Ca(OH)2 and the subsequent carbonation of the CaO intermediate. The relationship between the two component reaction processes was well illustrated by a consecutive shrinkage of the dual reaction interfaces of Ca(OH)2–CaO and CaO–CaCO3. The continuous supply of water vapor and CO2 to the CaO–CaCO3 interface from different directions was suggested to be the physico-geometrical advantageous feature of the carbonation of Ca(OH)2.
- This article is part of the themed collection: 2018 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...