Chiral differentiation of d- and l-isoleucine using permethylated β-cyclodextrin: infrared multiple photon dissociation spectroscopy, ion-mobility mass spectrometry, and DFT calculations†
Abstract
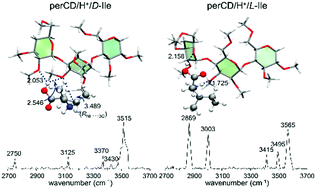
Chiral differentiation of protonated isoleucine (Ile) using permethylated β-cyclodextrin (perCD) in the gas-phase was studied using infrared multiple photon dissociation (IRMPD) spectroscopy, ion-mobility, and density functional theory (DFT) calculations. The gaseous protonated non-covalent complexes of perCD and D-Ile or L-Ile produced by electrospray ionization were interrogated by laser pulses in the wavenumber region of 2650 to 3800 cm−1. The IRMPD spectra showed remarkably different IR spectral features for the D-Ile or L-Ile and perCD non-covalent complexes. However, drift-tube ion-mobility experiments provided only a small difference in their collision cross-sections, and thus a limited separation of the D- and L-Ile complexes. DFT calculations revealed that the chiral distinction of the D- and L-complexes by IRMPD spectroscopy resulted from local interactions of the protonated Ile with perCD. Furthermore, the theoretical results showed that the IR absorption spectra of higher energy conformers (by ∼13.7 kcal mol−1) matched best with the experimentally observed IRMPD spectra. These conformers are speculated to be formed from kinetic-trapping of the solution-phase conformers. This study demonstrated that IRMPD spectroscopy provides an excellent platform for differentiating the subtle chiral difference of a small amino acid in a cyclodextrin-complexation environment; however, drift-tube ion-mobility did not have sufficient resolution to distinguish the chiral difference.


 Please wait while we load your content...
Please wait while we load your content...