Infrared photodissociation spectroscopy of ion-radical networks in cationic dimethylamine complexes†
Abstract
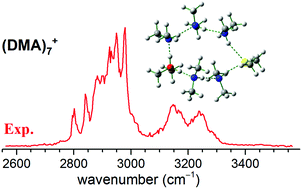
Infrared photodissociation spectroscopy was employed to establish the general trends in the stepwise growth motif of cationic dimethylamine (DMA)n+ (n = 4–13) complexes. Electronic structure calculations were performed to identify the structure of the low-lying isomers and to assign the observed spectral features. The results showed the preference of the formation of the proton-transferred (CH3)2NH2+ ion core. The (CH3)2NH2+–[(CH3)2N] ion-radical pair contact and the ion-radical separated pair could coexist at n = 4. The [(CH3)2N] radical is separated from the (CH3)2NH2+ ion core by one DMA molecule at n = 4–6 and by two or more DMA molecules in the larger clusters. This suggests that the (CH3)2NH2+–[(CH3)2N] ion-radical contact pair is not stable in the subsequent radiation-induced processes of DMA, and the [(CH3)2N] radical is released from the charged site in the cationic DMA networks.


 Please wait while we load your content...
Please wait while we load your content...