Different hydrogen bonding environments of the retinal protonated Schiff base control the photoisomerization in channelrhodopsin-2†
Abstract
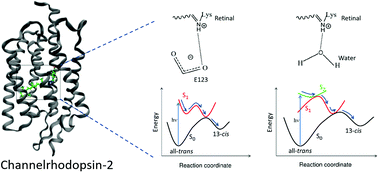
The first event of the channelrhodopsin-2 (ChR2) photocycle, i.e. trans-to-cis photoisomerization, is studied by means of quantum mechanics/molecular mechanics, taking into account the flexible retinal environment in the ground state. By treating the chromophore at the ab initio multiconfigurational level of theory, we can rationalize the experimental findings based on pump–probe spectroscopy, explaining the different and more complex scenario found for ChR2 in comparison to other rhodopsins. In particular, we find that depending on the hydrogen bonding pattern, different excited states are involved, hence making it possible to suggest one pattern as the most productive. Moreover, after photoisomerization the structure of the first photocycle intermediate, P5001, is characterized by simulating the infrared spectrum and compared to available experimental data. This was obtained by extensive molecular dynamics, where the chromophore is described by a semi-empirical method based on density functional theory. The results clearly identify which counterion is responsible for accepting the proton from the retinal Schiff base: the side chain of the glutamic acid E123.


 Please wait while we load your content...
Please wait while we load your content...