Optical transient absorption experiments reveal the failure of formal kinetics in diffusion assisted electron transfer reactions†
Abstract
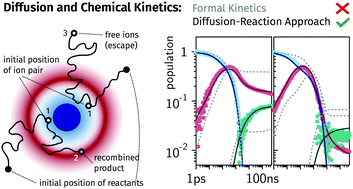
The ultimate goal of chemical kinetics is to understand why a given reaction is fast or not. To this end it is necessary to count on robust and experimentally well tested theories. One of the difficulties, long recognized in the study of bimolecular reactions, is the role of the molecular displacement, i.e. diffusion. Nonetheless the field is still lacking a compelling amount of case studies contrasting physical models to experiments. By performing transient absorption experiments on the photo-induced electron transfer reaction between perylene and N,N-dimethylaniline in liquid solutions over many orders of magnitude in time, we try to understand the factors determining the kinetics and yields of the full photocycle. We present a method to overcome potential pitfalls in the extraction of the relevant quantities, the transient populations, from the experimental data due to the changes in band shapes and positions. The results are compared to simulations of two different theories: a reaction–diffusion approach based on the encounter theories, and a formal kinetic scheme. We conclude that while the former explains the observed trends in the kinetics with quencher concentration and viscosity exceptionally well, the latter fails. Moreover the analysis of the data with the assistance of encounter theory unveils effects that otherwise would pass unnoticed. This approach and its results exemplify the path to follow in other condensed media whenever diffusion is involved.


 Please wait while we load your content...
Please wait while we load your content...