Why is the change of the Johari–Goldstein β-relaxation time by densification in ultrastable glass minor?†
Abstract
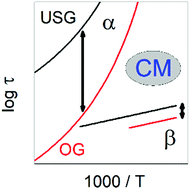
Ultrastable glasses (USG) formed by vapor deposition are considerably denser. The onset temperature of devitrification, Ton, is significantly higher than Ton or Tg of ordinary glass (OG) formed by cooling, which implies an increase of the structural α-relaxation time by many orders of magnitude in USG compared to that in OG at the same temperature. However, for a special type of secondary relaxation having properties strongly connected to those of the α-relaxation, called the Johari–Goldstein β-relaxation, its relaxation time in USG is about an order of magnitude slower than that in OG and it has nearly the same activation energy, Eβ. The much smaller change in τβ and practically no change in Eβ by densification in USG are in stark contrast to the behavior of the α-relaxation. This cannot be explained by asserting that the Johari–Goldstein (JG) β-relaxation is insensitive to densification in USG, since the JG β-relaxation strength is significantly reduced in USG to such a level that it would require several thousands of years of aging for an OG to reach the same state, and therefore the JG β-relaxation does respond to densification in USG like the α-relaxation. Here, we provide an explanation based on two general properties established from the studies of glasses and liquids at elevated pressures and applied to USG. The increase in density of the glasses formed under high pressure can be even larger than that in USG. One property is the approximate invariance of the ratio τα(Ton)/τβ(Ton) to density change at constant τα(Ton), and the other is the same ργ/T-dependence of τβ in USG and OG where ρ is the density and γ is a material constant. These two properties are derived using the Coupling Model, giving a theoretical explanation of the phenomena. The explanation is also relevant for a full understanding of the experimental result that approximately the same surface diffusion coefficient is found in USG and OG with and without physical aging, and ultrathin films of a molecular glass-former.
- This article is part of the themed collection: 2018 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...