Low temperature reaction dynamics for CH3OH + OH collisions on a new full dimensional potential energy surface†
Abstract
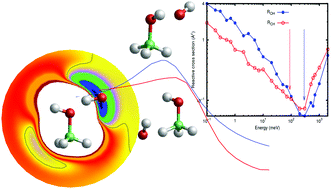
Is the rise of the rate constant measured in laval expansion experiments of OH with organic molecules at low temperatures due to the reaction between the reactants or due to the formation of complexes with the buffer gas? This question has importance for understanding the evolution of prebiotic molecules observed in different astrophysical objects. Among these molecules methanol is one of the most widely observed, and its reaction with OH has been studied by several groups showing a fast increase in the rate constant under 100 K. Transition state theory doesn't reproduce this behavior and here dynamical calculations are performed on a new full dimensional potential energy surface developed for this purpose. The calculated classical reactive cross sections show an increase at low collision energies due to a complex forming mechanism. However, the calculated rate constant at temperatures below 100 K remains lower than the observed one. Quantum effects are likely responsible for the measured behavior at low temperatures.


 Please wait while we load your content...
Please wait while we load your content...