Rationally designing mixed Cu–(μ-O)–M (M = Cu, Ag, Zn, Au) centers over zeolite materials with high catalytic activity towards methane activation†
Abstract
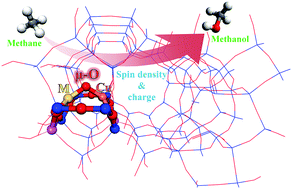
The direct conversion of methane to methanol on [Cu(μ-O)M]2+ (M = Cu, Ag, Zn, Au) bimetal centers in ZSM-5 zeolite is investigated using periodic density functional theory for the first time. Some conclusions are drawn: (1) methane activation on [Cu(μ-O)M]2+ (M = Cu, Ag, Zn, Au) in the ZSM-5 zeolite proceeds through radical-like transition states, and the ability for CH4 activation decreases in the sequence [Cu(μ-O)Ag]2+ > [Cu(μ-O)Au]2+ > [Cu(μ-O)Cu]2+ > [Cu(μ-O)Zn]2+. (2) There are two factors that can dramatically enhance C–H bond activation: a greater spin density and a less negative charge of the μ-O atom. (3) The angles ∠CuOM play a minor role in the reactivity difference among [CuOM]2+–ZSM-5 (M = Cu, Ag, Zn, Au). Our findings will provide insight into methane activation for designing highly effective catalysts applied in industrial processes.


 Please wait while we load your content...
Please wait while we load your content...