Increased stability of nitroxide radicals in ionic liquids: more than a viscosity effect†
Abstract
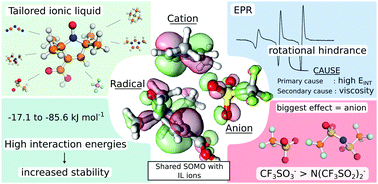
Radical stability has been subject to continuous research due to its importance in polymerization as well as in all-organic batteries. Recently, the SOMO–HOMO conversion was identified as the main factor in controlling the stability of distonic radicals, for which the negative charge resides on the same molecule. Based on this finding, the idea of ionic liquids stabilizing radicals was hypothesized in this study. A series of ionic liquids were tested in EPR measurements of the 3-carboxy-2,2,5,5-tetramethyl-pyrroline-1-oxyl. Unusually high rotational diffusion constants (τR), 4 times larger compared to conventional media such as dichloromethane (DCM), were recorded at room temperature. This finding could only be explained by a strong interaction existing between the radical and ionic liquid ions, which was confirmed with quantum chemical calculations, with interaction energies falling between −17.1 kJ mol−1 for tetramethylphosphonium tetrafluoroborate and −85.6 kJ mol−1 for 1,3-dimethylimidazolium triflate. Elevated temperature measurements performed at 80 °C reduced the viscosity of the ionic liquids to that of DCM, while the τR values remained relatively high, thus further confirming that the rotational hindrance occurred due to radical–ionic liquid interactions. The calculated interaction energies between the radical and ionic liquids ions were also found to correlate well with experimental rotational diffusion constants, thus offering us a valuable tool in tailoring ionic liquids for enhanced stability of nitroxide radicals. The findings of this study showcase the ability of ionic liquids to reduce reactivity of nitroxides without the need for any chemical modification of the radical.


 Please wait while we load your content...
Please wait while we load your content...