Impact of chirality on peculiar ibuprofen molecular dynamics: hydrogen bonding organization and syn vs. anti carboxylic group conformations†
Abstract
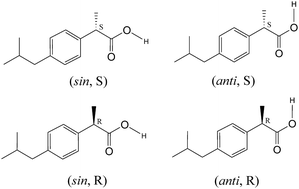
Studies of the impact of chirality on amorphous states are scarce. Here, we present combined dielectric relaxation spectroscopy (DRS) experiments and molecular dynamics (MD) simulation investigations of homochiral and racemic ibuprofen in the liquid, undercooled liquid and glassy states. The influence of chirality is particularly investigated on the syn and anti conformations of the –COOH moiety of the ibuprofen molecule and its link to the peculiar Debye-like dynamical process detected in this compound. Most of the studied properties are found to be nearly identical in the homochiral and racemic systems. But the polarity and intensity of the Debye-like process are clearly found to be more intense in the racemic mixture than in the enantiomerically pure ibuprofen. The difference is explained by the higher population of the anti conformation (with the higher dipole moment) and the lower population of hydrogen bonded cyclic dimers that can be transiently formed in the racemic mixture.


 Please wait while we load your content...
Please wait while we load your content...