Superatom chemistry: promising properties of near-spherical noble metal clusters
Abstract
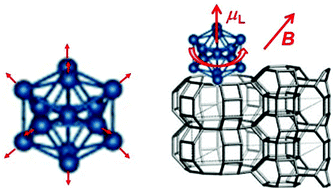
Properties of near-spherical metal clusters are best understood on the basis of the concept of conventional atoms. Their conduction electrons occupy cluster orbitals that remind of hydrogen-like orbitals since they have the same angular dependence. When populated with electrons, maxima in their ionization potentials and minima in electron affinities reveal the closing of shells in the same sense as for noble gases. This suggests that the periodic table of elements should be amended by a third dimension reflecting the number of atoms in a cluster of the element. In a bonded situation the symmetry of cluster atoms is broken, and the atomic orbital momentum is quenched to a large extent. However, if superatoms are axially symmetric, there are superatomic orbital angular moments that are locked along this symmetry axis. If their z-component is non-zero, this leads to large magnetic moments and to significant spin–orbit interactions, which greatly complicate spectroscopic observation. This magnetic interaction is anisotropic and may lead to hysteresis loops with corresponding blocking temperatures up to room temperature. The number of unpaired electrons in such a system is crucial, and it may be influenced by doping with different atoms or by chemical bonds to capping ligands. Stable superatom clusters with size-tuned, tailored band gaps and band edge energies may be attractive replacements for toxic or rare elements in photovoltaic cells or batteries; they form chemically inert and well-defined stoichiometric complexes with various ligands. This reminds of the established transition metal complexes and may lead to a novel branch of chemistry in which the central ion of organometallic complexes is replaced by a metal superatom.
- This article is part of the themed collections: PCCP Perspectives and 2018 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...