Cu(i) vs. Ru(ii) photosensitizers: elucidation of electron transfer processes within a series of structurally related complexes containing an extended π-system†
Abstract
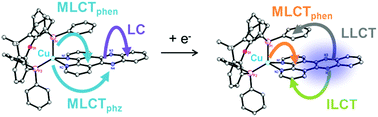
Heteroleptic Cu(I) complexes are a promising alternative towards traditional Ru(II) photosensitizers. In particular, Cu(I) complexes of the type [Cu(P^P)(N^N)]+, where N^N represents a diimine and P^P a bulky diphosphine ligand, are already successfully applied for photocatalysis, organic light-emitting diodes or dye-sensitized solar cells. Therefore, this study aims for the systematic comparison of three novel heteroleptic Cu(I) compounds, composed of xantphos (xant) as P^P ligand and different diimine ligands with an extended π-system in the backbone, with their structurally related Ru(II) analogues. In these Ru(II) photosensitizers [Ru(bpy)2(N^N)]2+ (bpy = 2,2′-bipyridine) the same N^N ligands were used, namely, dipyrido[3,2-f:2′,3′-h]quinoxaline (dpq) and dipyrido[3,2-a:2′,3′-c]phenazine (dppz). To gain an in-depth understanding of the photoinduced charge transfer processes, the photophysical features of these complexes and their electrochemically oxidized/reduced species were studied by a combination of UV-vis absorption, resonance Raman and spectroelectrochemistry. (TD)DFT calculations were applied to qualitatively analyze these measurements. As a result, the heteroleptic Cu(I) complexes exhibit comparable charge transfer properties to their Ru(II) analogues, i.e. upon visible light excitation they undergo a metal-to-ligand charge transfer to the diimine ligand(s). In contrast, the reduced Cu(I)– and Ru(II)–dppz complexes show considerably different electronic transitions. The singly reduced Cu(I)–dppz complexes are able to accumulate an additional electron at the phenanthroline moiety upon blue-light excitation, which is beneficial for multi-electron-transfer reactions. Upon low-energy light irradiation electronic transitions from the dppz− anion to the xant ligand are excited, which could shorten the lifetime of the photosensitizer intermediates in an unwanted way.


 Please wait while we load your content...
Please wait while we load your content...