Statistical thermodynamics for the unexpectedly large difference between disaccharide stereoisomers in terms of solubility in water†
Abstract
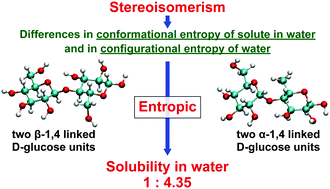
We unravel the physical origins of the large difference between cellobiose and maltose, which consist of two β-1,4 and α-1,4 linked D-glucose units, respectively, in terms of the solubility in water. We construct a thermodynamic theory where the chemical-potential difference between disaccharides in water and in vacuum is identified as the key free-energy function. Its energetic and entropic components are calculated for cellobiose and maltose by statistical-mechanical theories for solute hydration. The disaccharide structures are taken into account at the atomic level and a molecular model is adopted for water. Molecular dynamics simulations are used to account for the conformational fluctuation of a disaccharide molecule, which also enables us to estimate the conformational entropy. We show that the cellobiose/maltose solubility ratio calculated is in good agreement with the experimental value. The solubility becomes much lower for cellobiose due to conformational-entropy and water-entropy effects. The former effect is relevant to higher stability of the intramolecular hydrogen bond between oxygen atoms in the six-membered ring and in the neighboring hydroxyl group: the hydration alters the fluctuation of a molecular conformation to a larger or less regular one, but the degree of this alteration is smaller. The latter effect is attributed to more separation of two hydroxymethyl groups in a molecule, causing lower probability of the overlap of excluded volumes generated by the groups for water molecules. We suggest that physicochemical properties of disaccharides in water become variable depending on the stereoisomerism through hydration effects and the origins of the variety are entropic.


 Please wait while we load your content...
Please wait while we load your content...