Multi-scale theoretical approach to X-ray absorption spectra in disordered systems: an application to the study of Zn(ii) in water†
Abstract
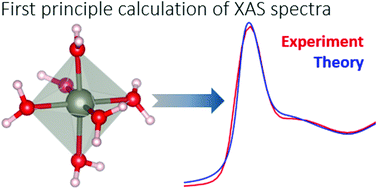
We develop a multi-scale theoretical approach aimed at calculating from first principles X-ray absorption spectra of liquid solutions and disordered systems. We test the method by considering the paradigmatic case of Zn(II) in water which, besides being relevant in itself, is also of interest for biology. With the help of classical molecular dynamics simulations we start by producing bunches of configurations differing for the Zn(II)–water coordination mode. Different coordination modes are obtained by making use of the so-called dummy atoms method. From the collected molecular dynamics trajectories, snapshots of a more manageable subsystem encompassing the metal site and two solvation layers are cut out. Density functional theory is used to optimize and relax these reduced system configurations employing a uniform dielectric to mimic the surrounding bulk liquid water. On the resulting structures, fully quantum mechanical X-ray absorption spectra calculations are performed by including core-hole effects and core-level shifts. The proposed approach does not rely on any guessing or fitting of the force field or of the atomic positions of the system. The comparison of the theoretically computed spectrum with the experimental Zn K-edge XANES data unambiguously demonstrates that among the different a priori possible geometries, Zn(II) in water lives in an octahedral coordination mode.


 Please wait while we load your content...
Please wait while we load your content...