Turn-off mode fluorescent norbornadiene-based photoswitches†
Abstract
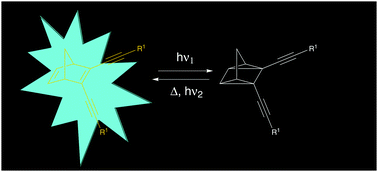
Single-molecule fluorescence emission of certain positive photochromic systems such as diarylethenes have been exploited for biological imaging and optical memory storage applications. However, there is a lack of understanding if negative photochromic systems can be used for such type of applications. Hence, to explore the potential of negative photochromic molecules for possible optical memory storage applications, we have here synthesized and studied a series of four norbornadiene–quadricyclane (NBD–QC) photoswitching molecules. These molecules feature either linearly conjugated or cross-conjugated pi-electron systems. Upon photoisomerization, the UV-vis absorption spectra of the molecules revealed a strong blue shift in the QC-form, with a photoisomerization quantum yield close to 80% for the cross-conjugated systems. In contrast, a strong intrinsic emission (up to Φf = 49%) for the linearly conjugated compounds in the NBD form was observed. Upon light-induced isomerization, the emission was completely turned off in the QC-form in all the compounds studied. Further, the robustness of the system was evaluated by performing several switching cycles. Under nitrogen, the emission can be turned off and recovered with almost no loss of emission. We also show that the QC-form can be photochemically triggered to convert back to the NBD-form using a low energy UV light (340 nm), allowing an all optical conversion to both species. The demonstrated properties can make the NBD–QC system attractive for potential applications such as optical memory storage devices.
- This article is part of the themed collection: 2018 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...