The involvement of carbon-centered radicals in the aging process of coals under atmospheric conditions: an EPR study†
Abstract
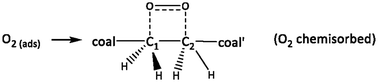
Coal exposed to an air atmosphere absorbs the atmospheric oxygen; this involves physical adsorption and chemisorption to form surface oxides (including hydro-peroxides). These weathering processes, which are denoted as LTO (Low Temperature Oxidation), decrease the calorific value of the coal and emit different gases such as carbon oxides (CO, CO2), water vapor, hydrogen (H2), and also some low-molecular-weight organic gases (C1–5). Some of these gases are toxic and flammable. The mechanism by which the molecular oxygen interacts with the coal macromolecule is thought to occur in several steps. The main concept is that a chain of radical reactions takes place; however, the exact underlying mechanism is not yet clear. We succeeded in identifying various carbon-centered radical species, depending on the coal rank and the degree of oxidation and suggested a new scheme for the formation of radicals via the coal oxidation process.


 Please wait while we load your content...
Please wait while we load your content...