From strong to weak NF bonds: on the design of a new class of fluorinating agents†
Abstract
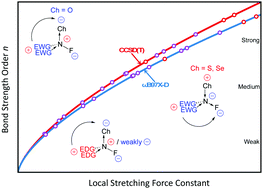
A set of 50 molecules with NF bonds was investigated to determine the factors that influence the strength of a NF bond, with the aim of designing a new class of fluorinating agents. The intrinsic bond strength of the NF bonds was used as bond strength measure, derived from local stretching NF force constants obtained at the CCSD(T)/aug-cc-pVTZ and ωB97XD/aug-cc-pVTZ levels of theory. The investigation showed that the NF bond is a tunable covalent bond, with bond strength orders ranging from 2.5 (very strong) to 0.1 (very weak). NF bond strengthening is caused by a combination of different factors and can be achieved by e.g. ionization. Whereas, the NF bond weakening can be achieved by hypervalency on the N atom, using a N→Ch (Ch: O, S, Se) donor–acceptor type bond with different electron-withdrawing groups. These new insights into the nature of the NF bond were used to propose and design a new class of fluorinating agents. Hypervalent amine-chalcogenides turned out as most promising candidates for efficient electrophilic fluorinating agents.
- This article is part of the themed collection: 2018 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...
