On-the-fly kinetics of hydrogen abstraction from polycyclic aromatic hydrocarbons by methyl/ethyl radicals†
Abstract
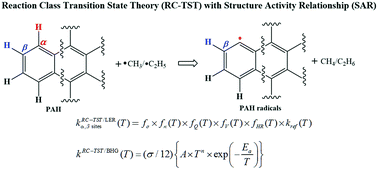
This work provides a rigorous procedure, within the framework of the Reaction Class Transition State Theory (RC-TST) and the Structure–Activity Relationship (SAR), for predicting reliable thermal rate constants on-the-fly for hydrogen abstraction reactions by methyl/ethyl radicals from Polycyclic Aromatic Hydrocarbons (PAHs) in a temperature range of 300–3000 K. All necessary RC-TST parameters were derived from ab initio calculations for a representative set of 36 reactions on which different error analyses and comparisons with available literature data were carried out. In addition to the good agreement between the RC-TST rate constants and the literature data, the detailed error analyses show that RC-TST/SAR, utilizing either the Linear Energy Relationship (LER) where only the reaction energy is needed or Barrier Height Grouping (BHG) where no additional data is needed, can predict the thermal rate constants for any reaction in the title reaction class with an average systematic error of less than 50% when compared to the explicit rate calculations. Therefore, the constructed RC-TST procedure can be confidently used to obtain reliable rate constants on the fly in an attempt to effectively construct detailed kinetic mechanisms for PAH-related fuels.


 Please wait while we load your content...
Please wait while we load your content...