Effective modulation of intramolecular ferromagnetic interaction of diradicals by functionalization of cross-conjugated coupler†
Abstract
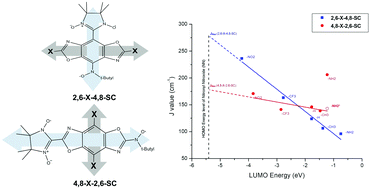
Cross-conjugated molecules are an interesting class of conjugated systems possessing a spatially separated HOMO and LUMO. Most previous studies have taken advantage of this property by using it in organic semiconductor applications. Herein, we undertake a new investigation on the use of this type of molecule, in particular benzo[1,2-d;4,5-d′]bisoxazole (BBO), as a coupler for organic diradicals. BBO has two sites available for adding a substituent and a spin center (SC) which are along its 4,8- and 2,6-axes. Functionalizations using electron donating (ED) and electron withdrawing (EW) groups were imposed to tune its FMOs and it was found that the longer 2,6-axis is an ideal site with a broader LUMO range via substituent effects. Diradicalization of these BBOs using nitronyl nitroxide (NN) and nitroxide (NO) as SCs was done using the remaining available axis. The calculated J values are linearly dependent on the LUMO energy of the coupler, but with 4,8-NH2-2,6-SC as an outlier. This exceptional case is related to 4,8-NH2-2,6-SC having the lowest BBO-NN dihedral angle. Moreover, the diradicals 4,8-X-2,6-SC (with X = H, NH2, CH3) have higher J values than 2,6-X-4,8-SC (with X = H, NH2, CH3), which is counterintuitive because the latter have a shorter coupling path. These diradicals are positioned to the right of the intersection of their trend lines, which implies that diradicals with LUMO values to the right of this intersection have the tendency to attain J values that are higher than those diradicals with a shorter coupling path. 4,8-NH2-2,6-SC even surpasses the projected JMax values which we associate with the highest attainable J values due to LUMO tuning via substituent effects. These results provide useful insights, especially into the interplay between the LUMO and the dihedral angle and how these affect magnetism in diradicals. In conclusion, we found that BBO can be a good candidate as an effective coupler for diradicals with tunable J values via incorporation of ED and EW groups. This first approach to studying the application of cross-conjugated molecules as couplers also paves the way for new candidates for the development of more effective diradical systems.


 Please wait while we load your content...
Please wait while we load your content...