The viscous consequence of different trends in clustering of 1,2-diol and 1,n-diol molecules
Abstract
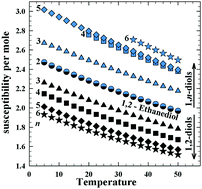
This paper presents the molecular basis for the quite different behavior of the viscosity of 1,2- and 1,n-diols in dependence of the length of the alkyl part of the molecules of these compounds. The experimental data on the dipolar orientational effects revealed a decidedly different role of that part of the molecules in creating a microstructure of both the hydrogen-bonded liquids. In the case of 1,n-diols, an increase in the alkyl radical length, i.e. an increasing of the distance between the OH groups within the molecule, highly stimulates molecular self-assembly in form of gradually longer and wider ribbon-like clusters. This effect yields a quite important increase in the viscosity of 1,n-diols as n increases. In the case of 1,2-diols, due to gradual separation of the hydrophilic and hydrophobic parts of the molecules, the situation is quite different. Two OH groups situated on one of the ends of the hydrocarbon radical form the clusters of a micelle-like shape, however, the dipole moment is not compensated. Along with an increase in the hydrocarbon part in 1,2-diol molecules, one only observes an increase in the intermolecular consolidation within the micelle-like entities. This manifests as a gradual decrease in the polarity of these clusters. So, actually, there are no relevant reasons for essential differences of viscosities in the series of 1,2-diols.


 Please wait while we load your content...
Please wait while we load your content...