Fast kinetics studies of the Lewis acid–base complexation of transient stannylenes with σ- and π-donors in solution†
Abstract
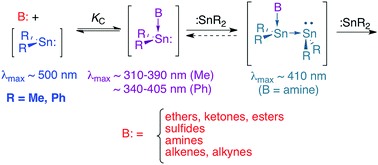
The Lewis acid–base complexation chemistry of dimethyl- and diphenylstannylene (SnMe2 and SnPh2, respectively) in hexanes solution has been studied by laser photolysis methods. Complexation of the two stannylenes with a series of nine O-, S-, and N-donors (including cyclic and acyclic dialkyl ethers and sulfides, a primary, secondary, and tertiary amine, ethyl acetate and acetone), two alkenes, and an alkyne proceeds rapidly and reversibly to generate the corresponding stannylene–donor Lewis pairs, which have been detected in each case by time-resolved UV-vis absorption spectroscopy. The complexes exhibit UV-vis absorption maxima in the range of λmax ∼ 310–405 nm depending on the donor and substitution at tin. Bimolecular rate constants for complexation (kC), which could be determined for 14 of the 24 Lewis pairs that were studied, were found to fall within a factor of four of the diffusional limit in all cases, with SnMe2 showing consistently higher reactivity than SnPh2. Equilibrium constants for complexation (KC) could be measured for all but one of the stannylene–π- and O-donor pairs, the values corresponding to (gas phase) binding free energies in the range of +1.1 to −3.9 kcal mol−1. Comparison of the experimental equilibrium constants for complexation of SnMe2 and SnPh2 with methanol and diethyl ether to those measured previously for the homologous silylenes and germylenes indicates that Lewis acidity decreases in the order SiR2 > SnR2 > GeR2 for both the dimethyl- and diphenyltetrylene series, the diphenyl derivatives exhibiting significantly stronger Lewis acidity in all three cases. The experimental trends in the binding energies and UV-vis spectra of the complexes are reproduced well by density functional theory (DFT) calculations, which have been carried out at the (TD)ωB97XD/def2-TZVP level of theory. The experimental data also show evidence of a reaction between tetramethyldistannene (Me2Sn![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) SnMe2, 4a) and amine donors, which is suggested to afford the corresponding amine-stabilized stannylidenestannylene structure. The mechanistic proposal is supported by DFT calculations of the complexation of 4a and SnMe2 with model O-, S- and N-donors.
SnMe2, 4a) and amine donors, which is suggested to afford the corresponding amine-stabilized stannylidenestannylene structure. The mechanistic proposal is supported by DFT calculations of the complexation of 4a and SnMe2 with model O-, S- and N-donors.


 Please wait while we load your content...
Please wait while we load your content...