Computational evidence for the degradation mechanism of haloalkane dehalogenase LinB and mutants of Leu248 to 1-chlorobutane†
Abstract
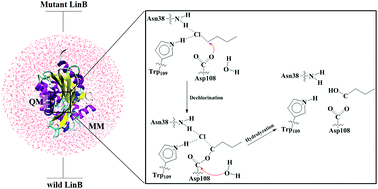
The catalytic degradation ability of the haloalkane dehalogenase LinB toward 1-chlorobutane (1-CB) was studied using a combined quantum mechanics/molecular mechanics (QM/MM) approach. Two major processes are involved in the LinB-catalyzed removal of halogens: dechlorination and hydrolyzation. The present study confirmed the experimentally proposed reaction path at the molecular level. Moreover, based on nucleophilic substitution mechanism (SN2 reaction), dechlorination was found to be the rate-determining step of the entire reaction process. In this study, the Boltzmann-weighted average barrier for dechlorination was determined to be 17.0 kcal mol−1, which is fairly close to the experimental value (17.4 kcal mol−1). The state of His107 and the influence of Leu248 on the dechlorination process were also explored. In addition, an intriguing phenomenon was discovered: the potential energy barrier decreased by 7.5 kcal mol−1 when the Leu248 residue was mutated into Phe248. This discovery might be of great help to design new mutant enzymes or novel biocatalysts.


 Please wait while we load your content...
Please wait while we load your content...