Ion speciation of lithium hexafluorophosphate in dimethyl carbonate solutions: an infrared spectroscopy study
Abstract
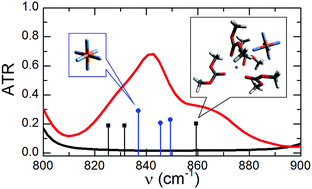
Solutions of lithium hexafluorophosphate (LiPF6) in linear organic carbonates play a significant role in the portable energy storage industry. However, many questions remain about the solution structure at the molecular-level. An atomic characterization of these solutions is important for determining their structure–property relations, which will allow for the rational design of new and improved lithium ion based energy storage technologies. In this study, a combination of infrared spectroscopies and density functional theory calculations was used to investigate the speciation of the lithium ion (free ion, solvent separated ion pair, contact ion pairs, and aggregates) in dimethyl carbonate solutions having concentrations ranging from 1 M to 3 M. The experimental data shows that at typical battery electrolyte concentrations the lithium ion exists predominantly as free ions and solvent separated ion pairs, but charged contact ion pairs are also present in small concentrations. In contrast, at high concentrations the lithium ion is present in aggregates, but a noticeable fraction remains present as free ions.


 Please wait while we load your content...
Please wait while we load your content...