Enhancement of CO2 adsorption on oxygen-functionalized epitaxial graphene surface under near-ambient conditions†
Abstract
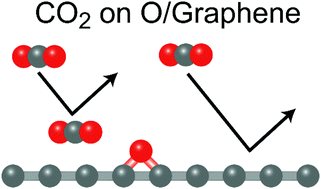
The functionalization of graphene is important in practical applications of graphene, such as in catalysts. However, the experimental study of the interactions of adsorbed molecules with functionalized graphene is difficult under ambient conditions at which catalysts are operated. Here, the adsorption of CO2 on an oxygen-functionalized epitaxial graphene surface was studied under near-ambient conditions using ambient-pressure X-ray photoelectron spectroscopy (AP-XPS). The oxygen-functionalization of graphene is achieved in situ by the photo-induced dissociation of CO2 with X-rays on graphene in a CO2 gas atmosphere. The oxygen species on the graphene surface is identified as the epoxy group by XPS binding energies and thermal stability. Under near-ambient conditions of 1.6 mbar CO2 gas pressure and 175 K sample temperature, CO2 molecules are not adsorbed on the pristine graphene, but are adsorbed on the oxygen-functionalized graphene surface. The increase in the adsorption energy of CO2 on the oxygen-functionalized graphene surface is supported by first-principles calculations with the van der Waals density functional (vdW-DF) method. The adsorption of CO2 on the oxygen-functionalized graphene surface is enhanced by both the electrostatic interactions between the CO2 and the epoxy group and the vdW interactions between the CO2 and graphene. The detailed understanding of the interaction between CO2 and the oxygen-functionalized graphene surface obtained in this study may assist in developing guidelines for designing novel graphene-based catalysts.


 Please wait while we load your content...
Please wait while we load your content...