Atomic hydrogen interactions with gas-phase coronene cations: hydrogenation versus fragmentation
Abstract
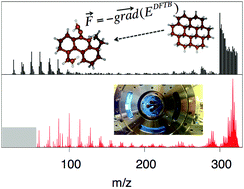
Sequential hydrogenation of polycyclic aromatic hydrocarbon (PAH) cations drives a gradual transition from a planar to a puckered geometry and from an aromatic to an aliphatic electronic structure. The resulting H-induced weakening of the molecular structure together with the exothermic nature of the consecutive H-attachment processes can lead to substantial molecular fragmentation. We have studied H attachment to gas-phase coronene cations in a radiofrequency ion trap using tandem mass spectrometry. With increasing hydrogenation, C2Hi loss and multifragmentation are identified as main de-excitation channels. To understand the dependence of both channels on H-exposure time, we have simulated the molecular stability and fragmentation channels of hydrogenated PAHs using a molecular dynamics approach employing potential energies determined by a density functional based tight binding method. As the coronene fragmentation patterns depend on the balance between energy deposition by H-attachment and the extent of cooling in between subsequent attachment processes, we investigate several scenarios for the energy distribution of hydrogenated PAHs. Good agreement between experiment and simulation is reached, when realistic energy distributions are considered.
- This article is part of the themed collection: 2018 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...