Aluminum and Fenton reaction: how can the reaction be modulated by speciation? A computational study using citrate as a test case
Abstract
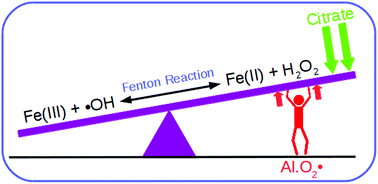
The pro-oxidant ability of aluminum is behind many of the potential toxic effects of this exogenous element in the human organism. Although the overall process is still far from being understood at the molecular level, the well known ability of aluminum to promote the Fenton reaction is mediated through the formation of stable aluminum–superoxide radical complexes. However, the properties of metal complexes are highly influenced by the speciation of the metal. In this paper, we investigate the effect that speciation could have on the pro-oxidant activity of aluminum. We choose citrate as a test case, because it is the main low-molecular-mass chelator of aluminum in blood serum, forming very stable aluminum–citrate complexes. The influence of citrate in the interaction of aluminum with the superoxide radical is investigated, determining how the formation of aluminum–citrate complexes affects the promotion of the Fenton reaction. The results indicate that citrate increases the stability of the aluminum–superoxide complexes through the formation of ternary compounds, and that the Fenton reaction is even more favorable when aluminum is chelated to citrate. Nevertheless, our results demonstrate that overall, citrate may prevent the pro-oxidant activity of aluminum: on one hand, in an excess of citrate, the formation of 1 : 2 aluminum–citrate complexes is expected. On the other hand, the chelation of iron by citrate makes the reduction of iron thermodynamically unfavorable. In summary, the results suggest that citrate can have both a promotion and protective role, depending on subtle factors, such as initial concentration, non-equilibrium behavior and the exchange rate of ligands in the first shell of the metals.


 Please wait while we load your content...
Please wait while we load your content...