Catalytic polymerization of naphthalene by HF/BF3 super acid: an ab initio density functional theory study†
Abstract
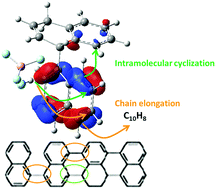
Mesophase pitch fabricated through polymerization of polycyclic aromatic hydrocarbons (PAHs) is highly aromatic and of high quality, and it can be used as a raw material to produce other carbon-based materials. Hydrofluoride/boron trifluoride (HF/BF3) is currently an efficient reagent to catalyze the PAH polymerization to produce mesophase pitch. In this study, density functional theory (DFT) calculations are performed to propose a mechanism for naphthalene catalytic polymerization using HF/BF3. The overall reaction mechanism can be conceptualized as having two stages: activation, followed by polymerization. During activation, HF/BF3 acts a proton donor to activate naphthalene, whose then-protonated form can promote the formation of a C–C bond with another naphthalene molecule via electrophilic addition. We also propose a catalyst recovery pathway, which can stabilize the intermediate products. In the polymerization stage, two types of pathways are proposed, those of chain elongation and intramolecular cyclization. According to the proposed catalytic mechanism in this study, the predicted mesophase product shows highly aliphatic hydrogens, which is consistent with the experimental results. We propose the full catalytic mechanism using DFT calculations. Our results provide a better understanding of how to develop novel and green catalysts, which can replace the HF/BF3 reagent in future applications.


 Please wait while we load your content...
Please wait while we load your content...