Temperature-programmed desorption studies of NH3 and H2O on the RuO2(110) surface: effects of adsorbate diffusion†
Abstract
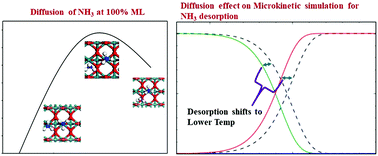
Temperature-programmed desorption (TPD) is one of the most straightforward surface science experiments for the determination of the thermodynamic and kinetic parameters of a reaction. In our previous study (J. Phys. Chem. C, 2013, 117, 6136–6142), we proposed a model combining DFT methods with microkinetics to investigate the TPD spectra of NH3 and H2O on the RuO2(110) surface. Although our model predicted both the physisorption and chemisorption peaks of both adsorbates in agreement with the experimental TPD spectra, it failed to explain the region between the physisorption and chemisorption areas and underestimated the intensity of the adsorbate in these areas. Hence, to improve our model, in this study, we considered the diffusion of adsorbates from the sub-monolayer to the second layer. Accordingly, we simulated the TPD spectra of both NH3 and H2O on the RuO2(110) surface using condensation approximation. Our results indicate that the diffusion barriers of the adsorbates at high coverage are smaller than their direct desorption energies. Hence, the diffusion of the adsorbates to the second layer from the sub-monolayer at higher coverage is kinetically favorable, which then desorb directly even at low temperatures. Furthermore, the simulated TPD spectra clearly depict the previous experimental results of both adsorbates after considering the diffusion.


 Please wait while we load your content...
Please wait while we load your content...