Thermodiffusion of repulsive charged nanoparticles – the interplay between single-particle and thermoelectric contributions†
Abstract
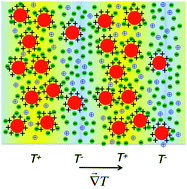
Thermodiffusion of different ferrite nanoparticles (NPs), ∼10 nm in diameter, is explored in tailor-made aqueous dispersions stabilized by electrostatic interparticle interactions. In the dispersions, electrosteric repulsion is the dominant force, which is tuned by an osmotic-stress technique, i.e. controlling of osmotic pressure Π, pH and ionic strength. It is then possible to map Π and the NPs’ osmotic compressibility χ in the dispersion with a Carnahan–Starling formalism of effective hard spheres (larger than the NPs’ core). The NPs are here dispersed with two different surface ionic species, either at pH ∼ 2 or 7, leading to a surface charge, either positive or negative. Their Ludwig–Soret ST coefficient together with their mass diffusion Dm coefficient are determined experimentally by forced Rayleigh scattering. All probed NPs display a thermophilic behavior (ST < 0) regardless of the ionic species used to cover the surface. We determine the NPs’ Eastman entropy of transfer and the Seebeck (thermoelectric) contribution to the measured Ludwig–Soret coefficient in these ionic dispersions. The NPs’ Eastman entropy of transfer ŜNP is interpreted through the electrostatic and hydration contributions of the ionic shell surrounding the NPs.
- This article is part of the themed collection: 2018 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...