Enhancing sodium ionic conductivity in tetragonal-Na3PS4 by halogen doping: a first principles investigation†
Abstract
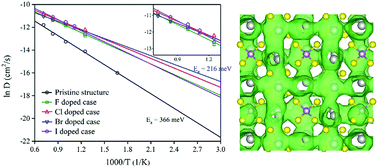
Tetragonal Na3PS4 (t-Na3PS4) has been demonstrated as a very promising candidate for a solid-state sodium-ion electrolyte with high Na ionic conductivity at ambient temperature. In this paper, we systematically investigated the Na ionic conductivity in pristine and halogen (F, Cl, Br, and I) doped tetragonal-Na3PS4 superionic conductors using first-principles calculations. The Na ionic conductivity of pristine t-Na3PS4 is calculated to be about 0.01 mS cm−1, while much higher Na ionic conductivities could be achieved by introducing Na ion vacancies via a halogen doping strategy. The calculated Na ionic conductivity of t-Na3PS4 doped with 1.56% Cl is 1.07 mS cm−1 at ambient temperature. Among different halogen-doped t-Na3PS4, Br-doped t-Na3PS4 shows the lowest activation energy and the highest Na ionic conductivity, which reaches 2.37 mS cm−1 at 300 K. The low activation energy and high Na ionic conductivity in Br-doped t-Na3PS4 are due to a relatively lower defect binding energy of the defect pair of halogen substitution and a Na ion vacancy. Our results suggest Br-doped t-Na3PS4 may serve as a very promising Na-ion solid-state superionic conductor.


 Please wait while we load your content...
Please wait while we load your content...