On the possibility of an Eley–Rideal mechanism for ammonia synthesis on Mn6N5+x (x = 1)-(111) surfaces†
Abstract
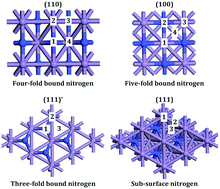
Recently we reported an Eley–Rideal/Mars–van Krevelen mechanism for ammonia synthesis on cobalt molybdenum nitride (Co3Mo3N). In this mechanism hydrogenation of activated dinitrogen occurs directly from the gas phase in a low barrier step forming a hydrazinylidene intermediate ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NNH2. In this paper we study whether such a mechanism of ammonia synthesis could occur on the (111) surface of another metal nitride, Mn6N5+x (x = 1), as this would explain the low-T ammonia synthesis activity of Co3Mo3N. We find that although N2 adsorbs more strongly than H2 on the (111) surface, having also examined the (110) and the (100) surface, N2 is not significantly activated when adsorbed in an end-on configuration. The hydrogenation reactions via an Eley–Rideal mechanism are all high barrier processes (>182 kJ mol−1) and therefore an Eley–Rideal mechanism for ammonia synthesis is predicted to not occur on this material unless there are high temperatures. Our study indicates that the fact that an Eley–Rideal/Mars–van Krevelen mechanism occurs on Co3Mo3N is a result of the stronger activation of dinitrogen at nitrogen vacancies when dinitrogen is adsorbed in an end-on configuration.
NNH2. In this paper we study whether such a mechanism of ammonia synthesis could occur on the (111) surface of another metal nitride, Mn6N5+x (x = 1), as this would explain the low-T ammonia synthesis activity of Co3Mo3N. We find that although N2 adsorbs more strongly than H2 on the (111) surface, having also examined the (110) and the (100) surface, N2 is not significantly activated when adsorbed in an end-on configuration. The hydrogenation reactions via an Eley–Rideal mechanism are all high barrier processes (>182 kJ mol−1) and therefore an Eley–Rideal mechanism for ammonia synthesis is predicted to not occur on this material unless there are high temperatures. Our study indicates that the fact that an Eley–Rideal/Mars–van Krevelen mechanism occurs on Co3Mo3N is a result of the stronger activation of dinitrogen at nitrogen vacancies when dinitrogen is adsorbed in an end-on configuration.


 Please wait while we load your content...
Please wait while we load your content...