Direct comparison between subnanometer hydration structures on hydrophilic and hydrophobic surfaces via three-dimensional scanning force microscopy†
Abstract
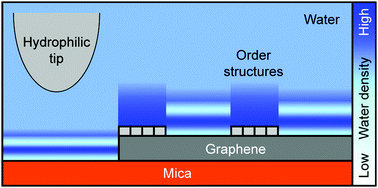
Investigating interfacial water ordering on solid surfaces with different hydrophobicities is fundamentally important. Here, we prepared hydrophilic mica substrates with some areas covered by mildly hydrophobic graphene layers and studied the resulting hydration layers using three-dimensional (3D) force measurements based on frequency-modulation atomic force microscopy. Hydration layers of 0.3–0.6 nm were detected on bare graphene regions; these layers were considerably larger than the spacing measured on mica (0.2–0.3 nm). On the graphene-covered regions, we also observed the formation of special ordered structures of adsorbates over time, on which, surprisingly, no prominent hydration layers were detected. Based on these findings, we present one possible scenario to describe the formation process of the ordered interfacial structures and the enhanced oscillation period in the force profiles. This work also demonstrates the capability and significance of 3D force measurements in probing hydration behaviors on a heterogeneous substrate with a lateral resolution smaller than several nanometers.


 Please wait while we load your content...
Please wait while we load your content...