Analysis of transition state stabilization by non-covalent interactions in organocatalysis: application of atomic and functional-group partitioned symmetry-adapted perturbation theory to the addition of organoboron reagents to fluoroketones†
Abstract
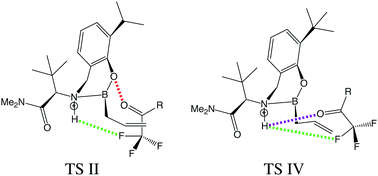
This work seeks to apply symmetry-adapted perturbation theory (SAPT) to the recent study of Hoveyda and co-workers [K. A. Lee et al., Nat. Chem. 2016, 8, 768] where an allyl addition to a ketone became enantioselective when the ketone was fluorinated. Through the application of atomic SAPT (A-SAPT) and functional-group SAPT (F-SAPT), the non-covalent interactions between specific atoms and functional groups in the transition states associated with the fluoroketone reactions can be quantified. Our A-SAPT analysis confirms that a H⋯F contact thought to enhance stereoselectivity shows a strong preference for one of the transition states leading to the experimentally observed product enantiomer. Other key atom–atom contacts invoked to rationalize relative transition state energies are also found to behave as expected based on chemical intuition and contact distances. On the other hand, hypothesized steric clashes between substrate phenyl or ortho-methyl phenyl groups and the catalyst are not supported by F-SAPT computations, and indeed, these are actually favorable π–π interactions.
- This article is part of the themed collection: 2018 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...